PFAS Testing Leadership
York-ALS Analytical is the leading laboratory in the Northeast for expert PFAS testing, blending regional expertise with national reach. Licensed in six states, our expanded laboratories, advanced instrumentation, and experienced technical teams consistently provide the fastest turnaround times (TATs) in the region.
As part of the global ALS laboratory network, we leverage world-class resources in PFAS research, regulatory tracking, and development of new testing solutions. This includes deploying the latest methods to ensure we stay at the forefront of evolving regulations and testing advancements for our clients.
The U.S. environmental testing market is projected to double in the next decade, driven by PFAS and emerging contaminants. York-ALS will offer the most comprehensive suite of PFAS testing services, and support clients through every phase of their projects—from initial investigation to remediation—with precision and reliability.
New York’s first PFAS Laboratory
YORK was the first-ever laboratory in NYC to be licensed by NELAC to analyze volatile organic compounds in soil and water, for TO-15 Air analysis and PFAS.
In 2017, York began managing PFAS sample analysis for clients in our existing Queens, NY facility, and in October 2018 we expanded it and opened the first and only dedicated PFAS laboratory in New York City. The new Emerging Contaminants laboratory was certified by the NYS DOH and approved in January 2019.


Your PFAS Team is Local
Our state-of-art PFAS laboratories are located in the heart of our service region, 90 miles apart in Queens, NY and Toms River, NJ. We mobilize our field service courier teams to meet clients’ bottle delivery, sampling and pickup needs across multiple states in the northeast region. Samples are never re-packaged and mailed, they get on our instruments fast and efficiently.

Your PFAS Labs are State-of-the-Art
York has invested aggressively and worked continuously with vendors to pioneer new technologies to become the most efficient PFAS laboratory network in the region. With a fleet of Aglient’s latest LC-MS/MS systems, and automated PromoChrom SPE extraction equipment, laboratory capacity is high so we can provide the fastest turnaround times.

Your PFAS Team is Innovative
We offer PFAS expertise that is second to none. The York-ALS senior technical team developed and implemented methods for preparation and analysis of drinking water, nonpotable water, and soil/solid matrices. Certified PFAS testing and data delivery is provided in less than 10 business days (including 1633 analysis.
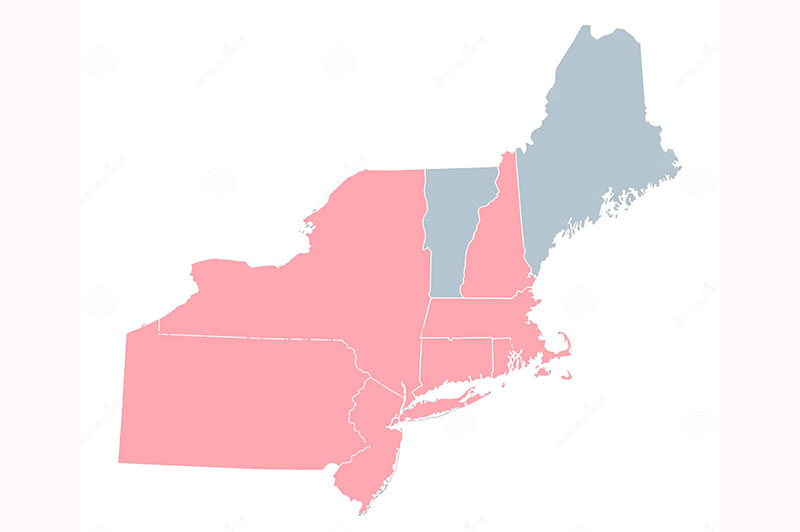
PFAS State Testing Certifications
Connecticut
Massachusetts
New Hampshire
New Jersey
New York
Pennsylvania
How We Do It
PFAS analysis can be broken down into three basic steps: extraction, concentration, and instrumental analysis. Once we’ve acquired our data, we can upload it to our Laboratory Information Management System (LIMS) for final reporting to you.
However, like any efficient analytical process, the devil is in the details. Having best practice sampling procedures at the outset will go a long way to obtaining the best data possible for your project. Once the samples are collected, we’ll handle the rest.

PFAS Sampling: Why it’s Important to You
Because PFAS compounds are ubiquitous, special sampling considerations should be taken prior to any sampling event.
Please see our sampling guides for detailed instructions to follow when collecting samples. We are analyzing samples down to part per trillion levels so cross-contamination prevention is an vital consideration.

Our Capacity
PFAS analysis is a highly technical process that requires specialized equipment and instrumentation. Across two locations, York-ALS has a high performing set of PromoChroms for automated sample preparation.
These advanced, robust extraction units allow for high throughput and consistent results. For analysis, York has the latest Agilent LC-MS/MS systems available, all operated by highly trained technical staff.

Future Considerations
As regulations unfold, reportable concentrations may be driven to lower levels. York-ALS is positioned to meet these needs.
The equipment and instrumentation in place at our advanced laboratories can achieve even greater sensitivity with further method development. This ability is crucial to staying ahead of the dynamic applications for PFAS.
PFAS Methods
York-ALS is certified for EPA 1633, EPA 537.1, EPA 533, and EPA 537m with certifications in six states and growing: New York, Connecticut, New Jersey, Pennsylvania, Massachusetts, and New Hampshire. Please refer to the table below for the PFAS compounds we’re able to analyze and their associated methods, and reach out to our client services team at clientservices@yorklab.com to obtain more information on our offerings in your specific state.
PFAS Compounds By Method
| Compound | EPA 537M* | EPA 537.1 | EPA 533 | EPA 1633 |
|---|---|---|---|---|
| Perfuoro-n-butanoic acid (PFBA) | ||||
| Perfuoro-n-pentanoic acid (PFPeA) | ||||
| Perfuorohexanoic acid (PFHxA) | ||||
| Perfuoroheptanoic acid (PFHpA) | ||||
| Perfuorooctanoic acid (PFOA) | ||||
| Perfuorononanoic acid (PFNA) | ||||
| Perfuorodecanoic acid (PFDA) | ||||
| Perfuoroundecanoic acid (PFUnA) | ||||
| Perfuorododecanoic acid (PFDoA) | ||||
| Perfuorotridecanoic acid (PFTrDA) | ||||
| Perfuorotetradecanoic acid (PFTeDA) | ||||
| Perfuorobutanesulfonic acid (PFBS) | ||||
| Perfuoro-1-pentanesulfonate (L-PFPeS) | ||||
| Perfuorohexanesulfonic acid (PFHxS) | ||||
| Perfuoro-1-heptanesulfonate (L-PFHpS) | ||||
| Perfuorooctanesulfonic acid (PFOS) | ||||
| Perfuorononanesulfonate (L-PFNS) | ||||
| Perfuoro-1-decanesulfonate (L-PFDS) | ||||
| Perfuorododecanesulfonic acid (PFDoS) | ||||
| 1H.1H.2H.2H-perfuoro-1-decanesulfonate (8:2 FTS) | ||||
| 1H.1H.2H.2H-perfuoro-1-hexanesulfonate (4:2 FTS) | ||||
| 1H.1H.2H.2H-perfuoro-1-octanesulfonate (6:2 FTS) | ||||
| Perfuoro-1-octanesulfonamide (FOSA) | ||||
| N-ethylperfuorooctanesulfonamedoacetic acid (N-EtFOSAA) | ||||
| N-methylperfuorooctanesulfonamdeoacetic acid (N-MeFOSAA) | ||||
| N-methylperfuorooctanesulfonamide (N-MeFOSA) | ||||
| N-ethylperfuorooctanesulfonamide (N-EtFOSA) | ||||
| N-methylperfuorooctanesulfonamidoethanol (N-MeFOSE) | ||||
| N-ethylperfuorooctanesulfonamidoethanol (N-EtFOSE) | ||||
| Hexafuoropropylene oxide-dimer acid (GenX/HFPO-DA) | ||||
| Dodecafuoro-3H-4.8-dioxanonanoate (ADONA) | ||||
| Nonafuoro-3.6-dioxaheptanoic acid (NFDHA) | ||||
| 9-Chlorohexadecafuoro-3-oxanone-1-sulfonic acid (9CL-PF3ONS) | ||||
| 11-chloroeicosafuoro-3-oxaundecane-1-sulfonic acid (11CL-PF3OUdS) | ||||
| Perfuoro-2-ethoxyethanesulfonic acid (PFEESA) | ||||
| Perfuoro-4-methoxybutanoic acid (PFMBA) | ||||
| Perfuoro-3-methoxypropanoic acid (PFMPA) | ||||
| 3-perfuoropropylpropanoic acid (3:3 FTCA) | ||||
| 2H.2H.3H.3H-perfuorooctanoic acid (5:3 FTCA) | ||||
| 3-perfuoroheptylpropanoic acid (7:3 FTCA) |



